- Laboratory of Neural Excitability and Neuromodulation
- Institute for Translational Brain Research,
- State Key Laboratory of Medical Neurobiology,
- MOE Frontiers Center for Brain Science,
- Department of Neurology, Huashan Hospital
- Fudan University
- Lab Head: Yousheng Shu
Email: yousheng@fudan.edu.cn
- The Fudan University Fenglin Campus, 131 Dong'an Road, Xuhui District, Shanghai
|
- Welcome to Yousheng Shu Lab
-
At the cellular level, equipped with our unique axon recording method, we study axon physiology and mechanisms that regulate the cell excitability, particularly the role of axonal ion channels and neurotransmitter receptors in regulating the generation and propagation of action potentials. At the circuit level, we study the structure and function of microcircuits within a brain region and neural circuits among distinct regions, dissect the mechanisms underlying the generation of network activities in different behavioral states. In addition, we study molecular, cellular, and circuit mechanisms of brain disorders, and develop new neuromodulation techniques for the treatment of diseases. See our research interest.
- Selected publications:
-
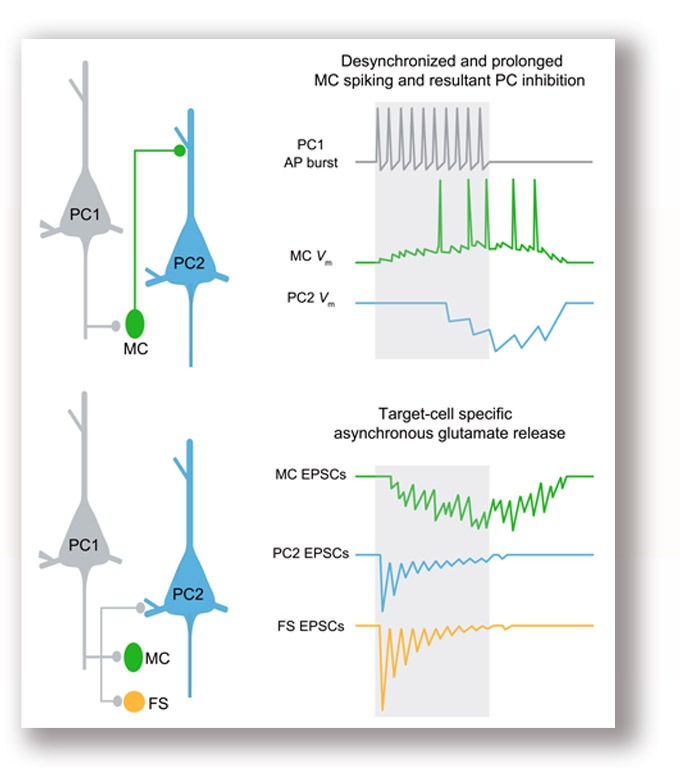 |
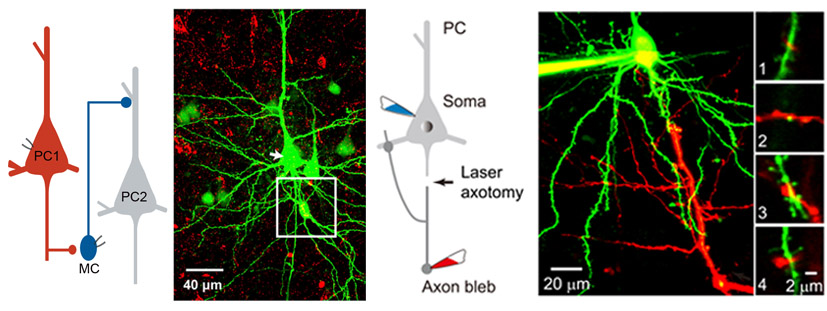
We performed dual whole-cell recordings from pyramidal cells (PCs) and nearby inhibitory interneurons in layer 5 of rodent neocortical slices. We found asynchronous release (AR) of glutamate occurs at PC output synapses onto Martinotti cells (MCs), causing desynchronized and prolonged firing in MCs and thus imprecise and long-lasting inhibition in neighboring PCs. These results highlight the effect of glutamate AR on the operation of microcircuits mediating slow recurrent inhibition, an important mechanism for controlling the timing and size of cortical inhibition. Deng et al., 2020 . |
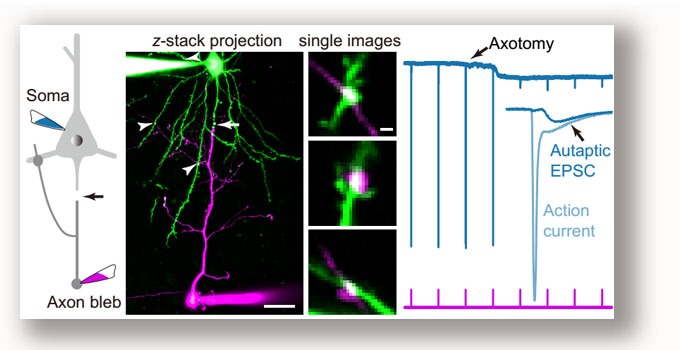 |
We performed 2-photon axotomy during simultaneous soma-axon recordings to isolate autaptic responses in neocortical pyramidal cells (PCs). We found that autapses (self-synapses) selectively form in subcortically projecting PCs and promote their burst firing by producing giant AMPA-only aEPSCs. Yin, Zheng, Ke, He et al., 2018 . |
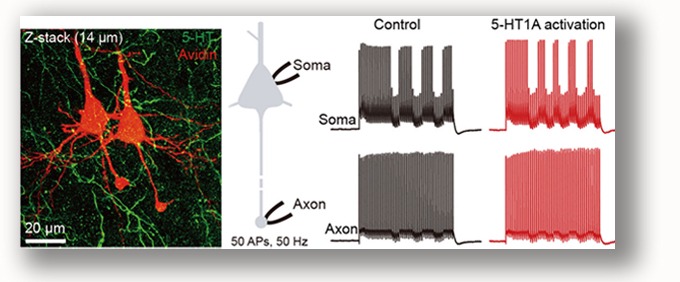 |
Activation of 5-HT1A receptors selectively inhibits Na+ channel subtype Nav1.2 but not Nav1.6, thus could decrease the success rate of action potential backpropagation toward the somatodendritic compartments, enhancing the segregation of axonal and dendritic activities. Yin et al., 2015 |
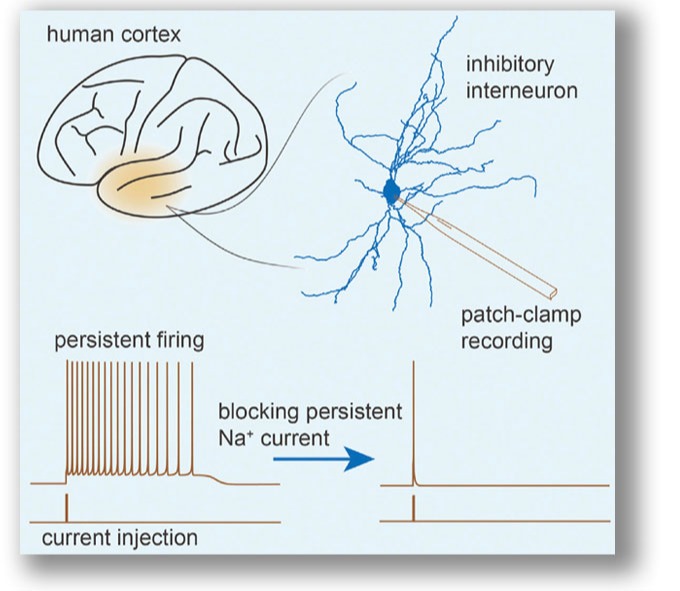 |
Our whole-cell recordings from human and monkey cortical slices reveal a distinct subpopulation of inhibitory interneuron with intrinsic persistent activity. Weak stimuli can turn on the persistent activity; however, strong stimuli can turn it off. This type of neuron could not be found in rodent cortical tissues, suggesting it could be unique to primates. This study is a good start to characterize cell types in human tissue with distinct physiological properties. Wang et al., 2015 . |
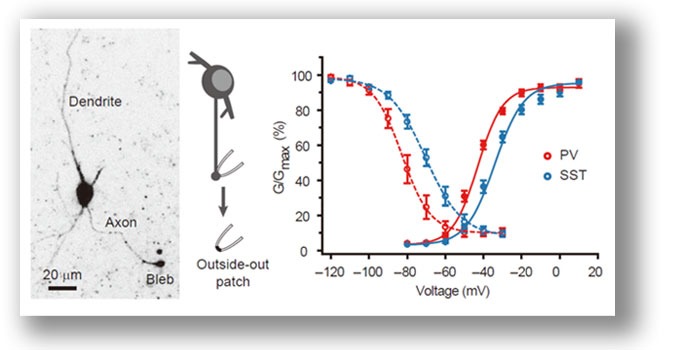 |
We performed patch-clamp recording from axon blebs of cortical inhibitory interneurons (PV and SST cells). Differences in voltage dependence of their AIS Na+ channels result from distinct distribution patterns of channel subtypes. Surprisingly, Nav1.2 is expressed by SST cells. Li et al., 2014 . |
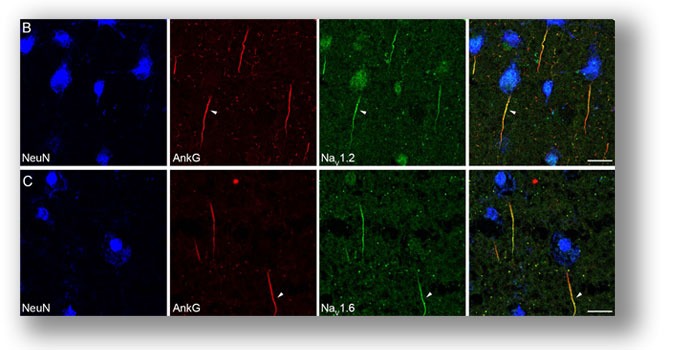 |
Immunostaining of Na+ channel subtypes in human cortical tissue. We found segregated distribution of proximal-Nav1.2 and distal-Nav1.6 at PC AIS. In addition to Nav1.6, the nodes of Ranvier in adult human cortex also express Nav1.2. We found no immunosignal of Nav1.1 at PC AIS. Tian et al.,2014 . |
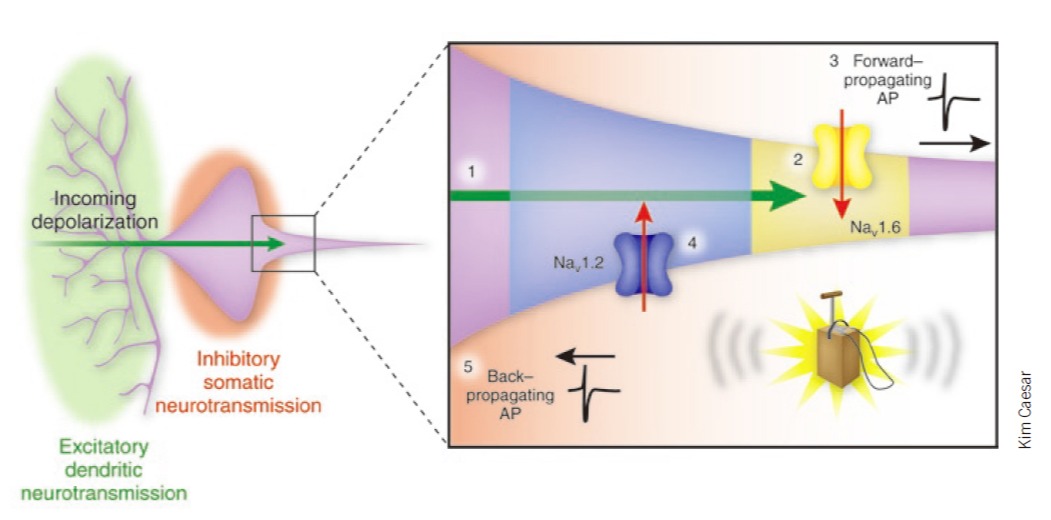 We found that Na+ channel subtype Nav1.6 determines action potential initiation and Nav1.2 promotes its backpropagation. Hu et al., 2009 . Also see comments: Dulla and Huguenard, 2009 . We found that Na+ channel subtype Nav1.6 determines action potential initiation and Nav1.2 promotes its backpropagation. Hu et al., 2009 . Also see comments: Dulla and Huguenard, 2009 .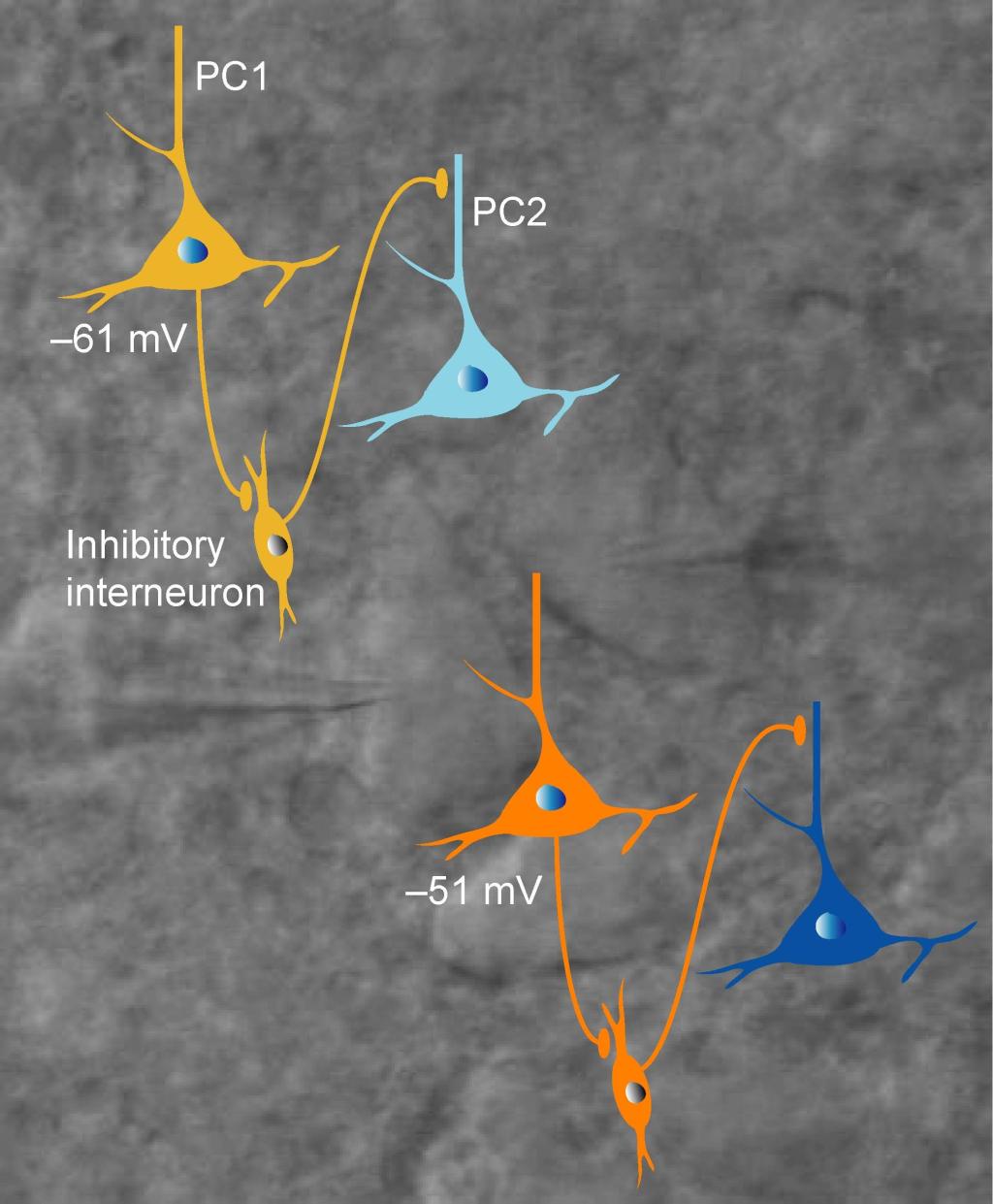 Membrane potential-dependent regulation of cortical recurrent inhibition. Zhu et al., 2011 . Also see . Membrane potential-dependent regulation of cortical recurrent inhibition. Zhu et al., 2011 . Also see .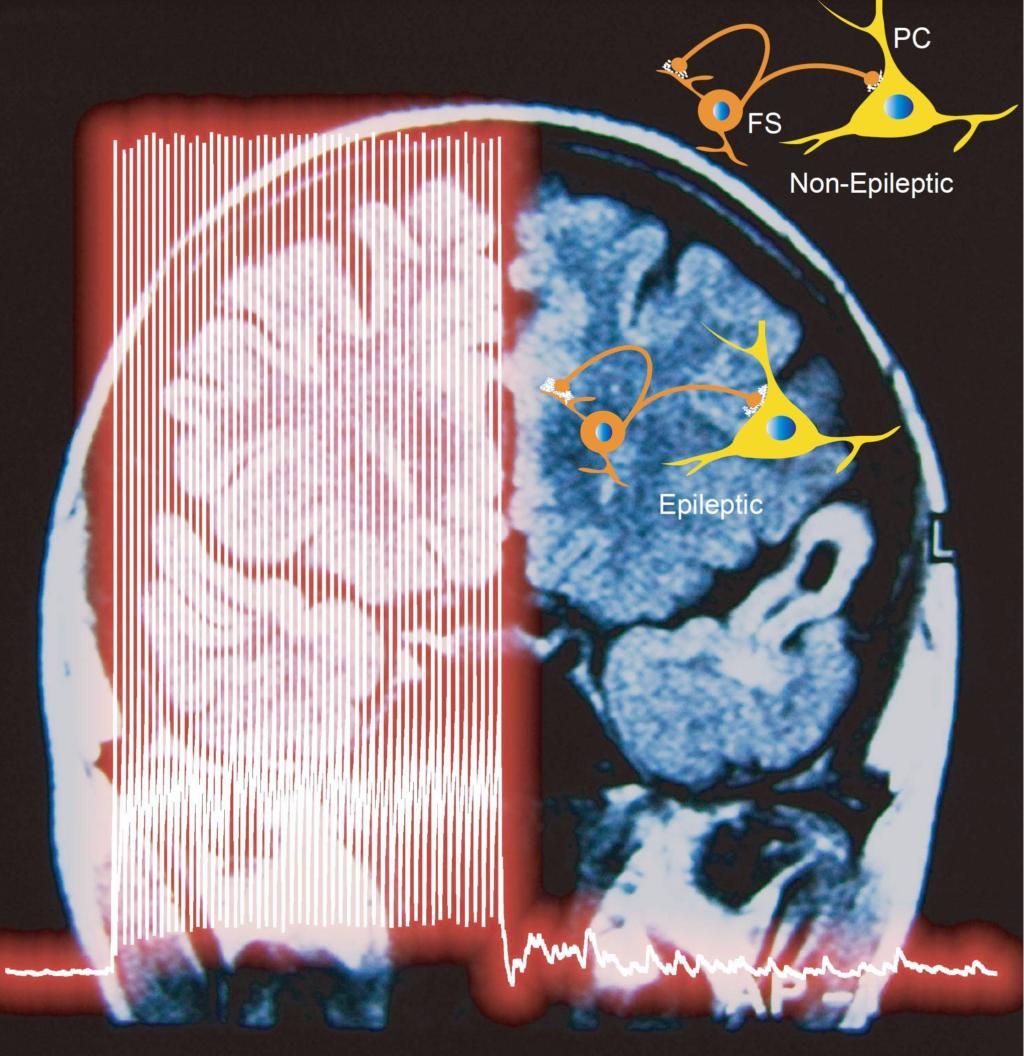 Asynchronous GABA release is upregulated in epileptic cortical tissue. Jiang et al., 2012 . Asynchronous GABA release is upregulated in epileptic cortical tissue. Jiang et al., 2012 .
|
